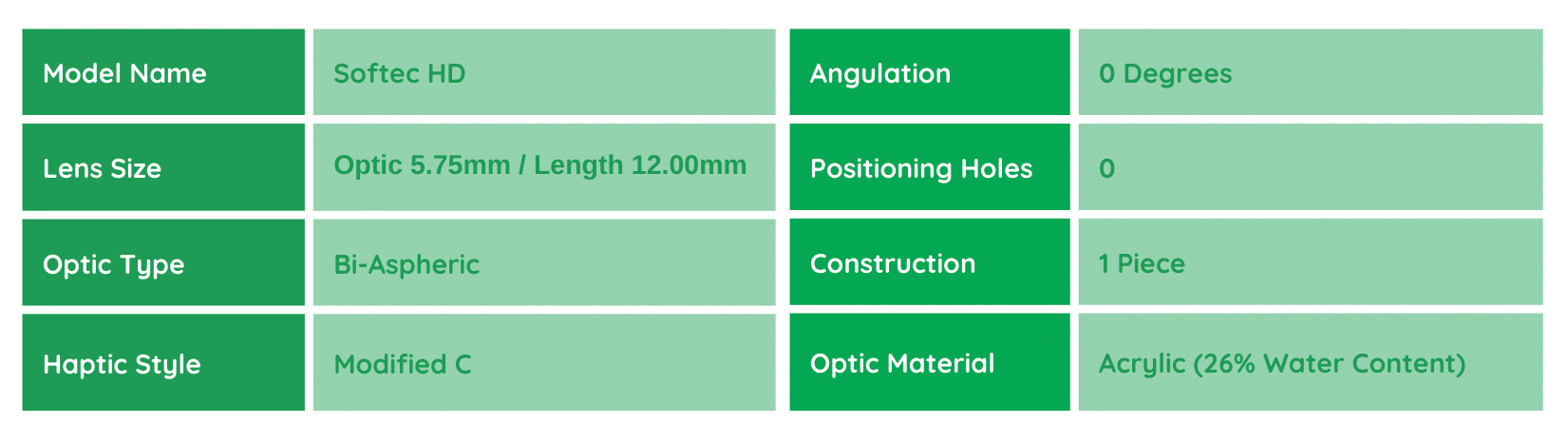
Softec HD
Softec HD
Intraocular lens (IOL) implantations are among the most successful and commonly performed surgical procedures globally, with capacity expected to double over the next two decades due to an aging population and increased demand for improved vision quality. IOL technology and innovation is critical in cataract surgeries, where the clouded lens is replaced with a synthetic one, restoring vision clarity.
The Lenstec Softec HD monofocal IOL is designed to meet the high expectations of modern cataract patients, offering an enhanced level of precision. A key advantage of the Softec HD IOL is its ability to address spherical aberration, a common issue in conventional monofocal IOLs. By incorporating a patented design on both the anterior and posterior surfaces, the lens achieves a single point of focus, resulting in sharper vision with no spherical aberration. This aspheric lens also addresses both negative and positive corneal aberration profiles, improves performance even with minor tilt or decentration, and reduces sensitivity to small pupils. The implant requires no changes in the surgeon’s skills or instrumentation, making it a seamless upgrade for surgeons and patients alike.
Given the growing demand for advanced vision solutions, the Softec HD’s ability to enhance precision and minimize optical aberrations makes it a standout choice in cataract surgery, particularly for patients seeking high quality outcomes.
Softec HD is available with the CLICK preloaded injector system, providing consistent and controlled lens delivery for seamless implantation. The system ensures precise, smooth lens placement, enhancing surgical efficiency and patient outcomes.
Read more to learn how Softec HD can enhance your practice and improve patient’s vision.
Not all products are approved in all countries. Contact us for availability.