Wow Nashville, You’re The Only “Ten I See!”
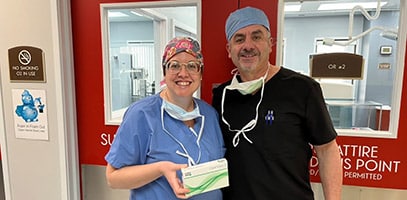
Wow Nashville, you’re the only “Ten I See!” 👁️ Dr. Jeffrey Horn at Vision for Life recently implanted 7 next generation ClearView™️ 3 multifocal lens implants with great results 🔥. It’s lookin’ pretty for patients in music city! Welcome to the ClearView family Dr. Horn!
Read MoreFirst ClearView 3 in Illinois!
Kudos to Kovach Eye and Dr. Marius Gradianu for being first in the state of Illinois to implant the ClearView™️ 3 Multifocal IOL. 👏 Great news for patients wanting new technology that provides a full range of vision, and low visual disturbances. 🎉Welcome to the ClearView family!
Read MoreAll Smiles in Sunny Sequim!
All smiles in Sunny Sequim! ☀️Congratulations to Dr. Max Psolka for implanting four ClearView™️ 3 lenses his first case week. Lenstec’s next gen multifocal technology, designed without concentric rings and in +0.25 diopter power increments, offers patients a full range of vision with low reported visual disturbances like glare and halos. Welcome to the ClearView…
Read MoreFamily Ties With ClearView Eyes
Family ties with ClearView Eyes! 👁️ Dr. Julia Song recently implanted Lenstec’s next gen ClearView™️ 3 multifocal IOL in her father with great results! In practice with her twin sister, Dr. Alice Song, at Southern CA Eye Physicians & Surgeons, this truly is a family affair. 🤙 Welcome Song family to the ClearView family!#southerncaeyephysiciansandsurgeons #drsongvision#lenstecinc…
Read MoreTennessee Triple in Kingsport!?
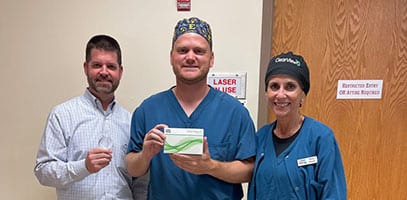
Tennessee Triple in Kingsport! 🎉 Great day in surgery with Dr. Craig Mitcham (Regional Eye Center) who implanted three ClearView™️3 multifocal lenses on his first day utilizing Lenstec’s next generation presbyopia correcting technology. 🙌 Way to go and welcome to the ClearView family Dr. Mitcham! #theregionaleyecenter #craigmitchammd #lenstecinc#clearviewlens #thinkoutsidetherings #norings#cataractsurgery
Read MoreHappy ClearView 3 Multifocal Patient
Happy ClearView™ 3 Multifocal Patient!Priceless post-op reaction after refractive lens exchangeperformed by Dr. Young Choi in Birmingham, AL. Click link below to see Facebook post from the Surgery Center: https://fb.watch/mIJuAOFYLl/?mibextid=HSR2mg
Read More“It’s All Good” in The Garden State
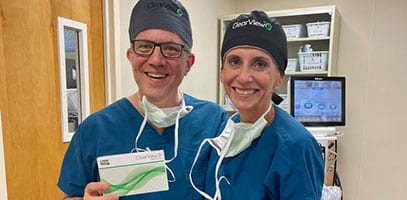
“It’s all good” in the Garden State🌹with Dr. Charles Crane recently implanting the ClearView™️3 multifocal IOL. He joins his colleague at Northern New Jersey Eye Institute in being able to offer patients the next generation in MIOL technology. Congratulations and welcome to the ClearView family Dr. Crane! 👏#northernnewjerseyeyeinstitute #charlescrane #lenstecinc #clearviewlens #thinkoutsidetherings #norings #catarcactsurgery
Read MoreOn The Map in Maine!
On the map in Maine! 🙌 Jordan Sterrer, MD (Eyecare Medical Group) recently implanted the first ClearView™️3 multifocal IOL in New England and is now able to offer his patients the next generation technology. 👁️ Welcome to the ClearView family Dr. Sterrer! #eyecaremedicalgroup #jordansterrer #lenstecinc#clearviewlens #norings #thinkoutsidetherings #cataractsurgery
Read MoreHigh Five for 5 ClearViews!
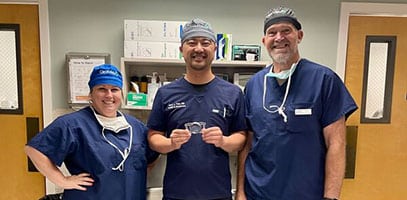
High five for 5 ClearViews! 👋🏻 Dr. Jack Tian(K2 Vision) is all smiles after implanting the first ClearView™️ 3 multifocal lenses in Washington State. The newest FDA approved MIOL has no concentric rings and provides patients a full range of vision and less nighttime glare and halos. Way to go Dr. Tian and welcome to the ClearView family!#k2visionrle…
Read MoreJuly 19th, 2023
On fire in Orlando! 🔥 Congratulations to Deepak Raja, MD (Remagin – Windermere, FL) for successfully implanting his first ClearView™️ 3 multifocal lens. With no concentric rings, the next generation MIOL provides patients a full range of vision with lower reported visual disturbances like glare and halos. Welcome to the ClearView Family Dr. Raja! 👏 #remagin…
Read MoreLearn How Lenstec Can Elevate Your Patient Care
Ready to learn more about how Lenstec can support your practice and improve patient outcomes? Contact us today to discuss your needs and explore our innovative solutions.